Research Projects
OESCA Supported Health Research Projects
**UPDATED 8/2/21** Grant 02536-MOU
Grant Title: Immunoprofiling to Combat Canine Immune Thrombocytopenia
Principal Investigator: Marjory Brooks, DVM
Research Institution: Cornell University
Original Project Description: Autoimmune disease develops in dogs when their immune system destroys normal healthy cells in the body. Immune thrombocytopenia (ITP) is a serious bleeding disorder that results from immune destruction of platelets, small blood cells that play a critical role in preventing bruising and bleeding after injury. Old English Sheepdogs and Cocker Spaniels appear to have a susceptibility to ITP, however, ITP afflicts all dogs regardless of breed. Dogs with ITP develop bruises and, in the most severe cases, may bleed from the intestinal and urinary tract or have fatal blood loss. Fortunately, most dogs survive ITP, but may relapse months to years after a first episode. The treatment of ITP involves protracted courses of potent immunosuppressive drugs that impact quality of life for both dog and owner. This study will use a genetic approach to understand what causes ITP. The investigators will identify laboratory markers that predict bleeding severity to aid veterinarians in treatment selection. The goals of this research are to improve ITP diagnosis and predictions of relapse, leading to targeted therapies that minimize treatment side effects.
Funding for the research is provided through the collaborative efforts and generosity of the Old English Sheepdog Club of America and English Cocker Spaniel Club of America Health and Rescue Organization. The AKC Canine Health Foundation supports the funding of this effort and will oversee grant administration and scientific progress.
Publications: Makielski, K. M., Brooks, M. B., Wang, C., Cullen, J. N., O’Connor, A. M., & LeVine, D. N. (2018).
Development and implementation of a novel immune thrombocytopenia bleeding score for dogs.
Journal of Veterinary Internal Medicine, 32(3), 1041–1050. https://doi.org/10.1111/jvim.15089
An original report “Preliminary Evaluation of a Flow Cytometric Assay with Controls for Detection of
Platelet Bound Antibodies in Canine Immune Thrombocytopenia ” has been resubmitted for review to
the Journal Veterinary Clinical Pathology.
Presentations: Cornell University: Internal medicine seminar 01/18/19: “Canine Immune Thrombocytopenia”.
An abstract was presented at the 2020 ACVIM forum:
*Barchilon M, Viall AK, Gagne JE, Phalen EE, Boggiatto B, Schaut RE, Jeffery U, Brooks MB, LeVine DN.
Immune profiles of Cocker Spaniels and Old English Sheepdogs, breeds predisposed to autoimmune
blood disorders. E-poster presentation, Research Abstract Program of the 38th ACVIM Forum on
Demand. Virtual. July 2020. Journal of Veterinary Internal Medicine. 2020; 2020; 34: 2905.
Report to Grant Sponsor from Investigator:
We have now reached our target numbers of dogs for both study aims! Our requests for samples for
genetic studies from Old English Sheepdogs and Cocker Spaniels yielded 25 control samples (aged dogs
with no blood disorders) and 23 ITP cases. These samples will be analyzed along with 78 samples from
other ITP cases representing many other breeds to screen for genetic markers of autoimmune platelet
destruction.
For our second study aim, we now have a total of 102 cases. After the recruitment phase ends in
August, we will begin testing banked samples from these dogs to measure inflammatory markers. This
data will then be combined for analysis with the dogs’ clinical signs of bleeding, treatment and
transfusion history, and overall survival. Our early findings are showing patterns of laboratory test
abnormalities that seem to differentiate dogs with “true” autoimmune platelet destruction from those
with an underlying disease process. Our additional data analyses will help determine if we can find
tests that predict which dogs have the most severe forms of ITP to better individualize their therapy.
This will spare dogs with mild disease the side effects of aggressive treatment while letting
veterinarians know that they need to more aggressively treat those with markers of severe disease.
Downloadable and Printable Summary
Grant 02536-MOU
Grant Title: Immunoprofiling to Combat Canine Immune Thrombocytopenia
Principal Investigator: Marjory Brooks, DVM and Dana LeVine, DVM, PhD
Research Institution: Cornell University and Iowa State University
Original Project Description: Autoimmune disease develops in dogs when their immune system destroys normal healthy cells in the body. Immune thrombocytopenia (ITP) is a serious bleeding disorder that results from immune destruction of platelets, small blood cells that play a critical role in preventing bruising and bleeding after injury. Old English Sheepdogs and Cocker Spaniels appear to have a susceptibility to ITP, however, ITP afflicts all dogs regardless of breed. Dogs with ITP develop bruises and, in the most severe cases, may bleed from the intestinal and urinary tract or have fatal blood loss. Fortunately, most dogs survive ITP, but may relapse months to years after a first episode. The treatment of ITP involves protracted courses of potent immunosuppressive drugs that impact quality of life for both dog and owner. This study will use a genetic approach to understand what causes ITP. The investigators will identify laboratory markers that predict bleeding severity to aid veterinarians in treatment selection. The goals of this research are to improve ITP diagnosis and predictions of relapse, leading to targeted therapies that minimize treatment side effects
Funding for the research is provided through the collaborative efforts and generosity of the Old English Sheepdog Club of America and English Cocker Spaniel Club of America Health and Rescue Organization. The AKC Canine Health Foundation supports the funding of this effort and will oversee grant administration and scientific progress.
Publications: Makielski, K. M., Brooks, M. B., Wang, C., Cullen, J. N., O’Connor, A. M., & LeVine, D. N. (2018). Development and implementation of a novel immune thrombocytopenia bleeding score for dogs. Journal of Veterinary Internal Medicine, 32(3), 1041–1050. https://doi.org/10.1111/jvim.15089 A draft publication “A Flow Cytometric Assay and Preparation of Assay Controls for Detection of Platelet Bound Antibodies and Platelet Membrane Abnormalities in Clinical Studies of Canine Immune Mediated Thrombocytopenia” is in preparation and will be submitted for review to Veterinary Clinical Pathology.
Presentations: Cornell University: Internal medicine seminar 01/18/19: “Canine Immune Thrombocytopenia”.
An abstract is being presented as an eposter at the 2020 ACVIM virtual on-demand forum “Immune Profiles of Cocker Spaniels and Old English Sheepdogs, Breeds Predisposed to Autoimmune Blood Disorders ” (authors: Barchilon M, Viall A, Gagne J, Phalen E, Boggiatto P, Schaut R, Jeffery U, Brooks MB, LeVine DN.)
Another abstract has been submitted for presentation at the the 62nd American Society of Hematology Annual Meeting and is currently under review.
Report to Grant Sponsor from Investigator:
We have now reached 80% of the target number of dogs to perform ITP genetic studies and our second batch of samples is now being analyzed. This total includes 43 dogs from breeds with known susceptibility to immune blood disorders (19 Old English Sheepdogs, 24 American and English Cocker Spaniels). We are still in need, however, of more samples from a comparison group of “aged” dogs in these breeds with no prior history of blood disorders. For our second aim of biomarker development, we have now reached 70% of our target number of dogs. Early analysis of data is beginning to show patterns of abnormalities to help differentiate cases with an underlying disorder from dogs with “true” autoimmune platelet destruction.
Downloadable and Printable Summary
Grant 02610-A
Grant Title: The Role of Motilin Signaling in Canine Osteoarthritis
Principal Investigator: Li Zeng, PhD
Research Institution: Tufts University
Original Project Description: Osteoarthritis is a devastating disease characterized by joint pain and immobility and while it is highly prevalent in dogs, there is no optimal treatment for this disease. The goal of this study is to design strategies to prevent osteoarthritis progression and improve the quality of life for dogs. A central feature for osteoarthritis is the destruction of joint cartilage, a tissue that normally serves as a cushion between bones. Without this cushion, there is increased friction at the joint, causing mechanical stress and accelerating joint degeneration. One treatment strategy is to combat inflammation, because inflammation results in joint cartilage loss and is a key component in the pathogenesis of osteoarthritis. In preliminary studies, the investigators found that the hormone motilin has an anti-inflammatory activity that has not been previously reported. Their hypothesis is that motilin protects the canine joint against inflammation and improves the health of the cartilage in osteoarthritis. Outcomes of this research may benefit both dogs and humans suffering from osteoarthritis.
Publications: None at this time.
Presentations: We presented part of the data (whole joint culture) in a local symposium (Center for Skeletal Research, MGH, in May 2019).
Report to Grant Sponsor from Investigator:
Osteoarthritis causes severe immobility in dogs, especially in certain large breeds, suggestive of an underlying genetic mechanism. In addition to the genetic component, repeated use causes mechanical stress which further results in chronic joint inflammation and articular cartilage degeneration. This creates a significant emotional and economic burden to the society. Thus, there is an urgent need to find a therapy to halt osteoarthritis progression.
We have been investigating the role of motilin on canine cartilage degeneration to understand the genetic regulation of joint stability and in search of a potential treatment option for osteoarthritis. Motilin, a hormone that enhances GI movement, has been shown to have anti-inflammatory activity. With the support of this grant, we have established two systems in the lab to analyze canine cartilage, including the culture conditions that enable the survival of cells in the joint. Our data suggests a reduction of motilin receptor in cartilage from osteoarthritis dogs, and an effect of motilin to modify chondrocytes’ response to inflammation, which may be unique to canines, pointing to the importance of studying osteoarthritis and inflammation specifically in dogs, rather than adopting treatments from human and mouse studies.
Downloadable and Printable Summary
Eye Disease - Progressive Retinal Atrophy (PRA)
Grant Title: Investigation of the Genetic Mutations Underlying Progressive Retinal Atrophy
Principal Investigator: Simon Petersen-Jones, PhD
Research Institution: Michigan State University
Purpose: To identify the gene mutation that causes PRA in the Old English Sheepdog and develop a screening test to identify carriers and affected PRA dogs. Such a test could enable the eradication of PRA in the breed.
You Can Participate: If you own a PRA diagnosed dog, help researchers by collecting and submitting DNA samples from the PRA dog and immediate relatives.
If you have DNA stored on a dog who was diagnosed with PRA, contact Dr. Petersen-Jones. Stored blood can also contain valuable information.
Contact:
Dr. Petersen-Jones
517-353-3278
peter315cvm.msu.edu
Over 30 breeds have a screening test for PRA.
Grant 02052: Defining the Mechanism of Severe, Life-Threatening Bleeding Disorders in Dogs
Grant Title: Grant 02052: Defining the Mechanism of Severe, Life-Threatening Bleeding Disorders in Dogs
Principal Investigator: Dr. Dana N. LeVine, DVM, PhD
Research Institution: Iowa State University
Start - End Dates: 2/1/2014 - 1/31/2015
Original Project Description:
Immune thrombocytopenia (ITP) is a common bleeding disorder in dogs. It occurs when the immune system destroys the body's own platelets, blood cells that prevent hemorrhage. The resulting lack of platelets in some dogs causes mild bruising and in others causes severe, lifethreatening hemorrhage.
Veterinarians do not understand what triggers ITP and cannot predict its severity. Consequently, all ITP patients are treated with potent medications that suppress the entire immune system. Many dogs experience treatment side-effects including excessive thirst and urination, ulcers, weight gain, and recurrent infections. For some dogs, the side-effects, rather than ITP, prove fatal.
Our first aim investigates the specific causes of ITP. We will identify an ITP disease profile by measuring immune cells and proteins that may be involved platelet destruction. We will also look for genes associated with the disease in Old English Sheepdogs and Cocker Spaniels, since these breeds are especially prone to ITP. These tests will suggest the specific immune and genetic causes of ITP, so targeted drugs can be developed that suppress just these mechanisms, not the whole immune system.
Our second aim is designed to find laboratory markers that predict bleeding severity. Using these markers, veterinarians will be able to reserve aggressive treatment for only those dogs at risk for significant blood loss.
Together, these aims will benefit ITP patients through individualized therapy that matches treatment intensity with disease severity. Discovery of the immune and genetic causes of ITP will not only improve disease treatment, but ultimately help to prevent it.
Grant Objectives:
- To investigate the specific causes of immune thrombocytopenia (ITP).
- To find laboratory markers that predict bleeding severity.
Report to Grant Sponsor from Investigator:
Our project aims to investigate the specific causes of ITP by identifying an ITP disease profile by measuring immune cells and proteins that may be involved platelet destruction. We also plan to look for genes associated with the disease in Old English Sheepdogs (OES) and Cocker Spaniels (CS), since these breeds are especially prone to ITP.
In this initial study period we have established the infrastructure for a two-pronged attack on canine ITP:
1. Targeted evaluation of breeds at risk: OES and Cocker spaniels
Through the efforts of many dedicated breeders, we have received samples from ITP affected dogs and controls for DNA banking and genetic analyses. We have already found some significant differences in the immune cell profiles of Cocker Spaniels compared to other breeds of dogs. We are now exploring these differences to determine whether they make Cockers at particular risk for autoimmune diseases. We are also looking for similar patterns in each new case of ITP as they are enrolled in our study.
2. In-depth immunoprofiling of newly diagnosed ITP cases
We have set up a multi-institution collaboration among three veterinary academic and referral centers (Cornell University, Iowa State University, and Cornell University Veterinary Specialists) to enroll active ITP cases. We have developed a novel in depth immune profile system and a new bleeding severity scoring system. The new bleeding score system is important to standardize grading of each dog’s clinical status and relate physical exam findings to laboratory test results. Ultimately, the markers of disease severity that we identify will help veterinarians reserve aggressive treatment for only those ITP affected dogs at risk for significant blood loss.
Together these endeavors will provide a better understanding of the cause of ITP and new approaches to treatment, including individualized therapy of ITP patients based on disease
severity.
Grant Abstract: 2052 MY1 Summary
*** ARCHIVAL HISTORY OF PREVIOUS GRANTS ***
01609: Probiotic VSL# 3 Reduces Enteritis in Dogs with Inflammatory Bowel Disease
Grant Title: 01609: Probiotic VSL# 3 Reduces Enteritis in Dogs with Inflammatory Bowel Disease
Principal Investigator: Dr. Albert E. Jergens, DVM, PhD
Research Institution: Iowa State University
Start - End Dates: 1/1/2012 - 12/31/2014
Original Project Description:
Idiopathic inflammatory bowel disease (IBD) is a common cause of chronic gastrointestinal disease in dogs. Accumulating evidence in human IBD and animal models suggests that imbalances in composition of the intestinal microbiota contribute to the pathogenesis of chronic intestinal inflammation. Recent studies have also shown that dogs with IBD have distinctly different duodenal microbial communities compared to healthy dogs. Current treatments for IBD include the administration of nonspecific anti-inflammatory drugs which may confer serious side effects and do not address the underlying basis for disease, namely, altered microbial composition. Use of probiotics (viable, non-pathogenic bacteria that exert health benefits beyond basic nutrition) offers an attractive, physiologic, and non-toxic alternative to shift the balance to protective species and treat IBD. The aim of the proposed study is to investigate the clinical, microbiologic, and anti-inflammatory effects of probiotic VSL#3 in the treatment of canine IBD. We hypothesis that VSL#3 used as an adjunct to standard therapy (i.e., elimination diet and prednisone) will induce a beneficial alteration of enteric bacteria leading to induction and maintenance of remission in dogs with IBD. A randomized, controlled clinical trial of 8 weeks duration will assess the efficacy of standard therapy + probiotic versus standard therapy alone. There is a need for additional data to be generated to provide proof of efficacy in probiotic therapy before these agents can be applied to widespread clinical use. These studies will also provide highly relevant insight into the anti-inflammatory effects of probiotics for treatment of human and canine IBD.
Grant Objectives:
To determine the clinical, microbiologic, and anti-inflammatory effects of probiotic VSL #3 in the treatment of canine IBD.
Report to Grant Sponsor from Investigator:
Idiopathic inflammatory bowel disease (IBD) is a common cause of chronic gastrointestinal disease in dogs. Accumulating evidence in human IBD and animal models suggests that imbalances in composition of the intestinal microbiota contribute to the pathogenesis of chronic intestinal inflammation. Recent studies in dogs with IBD have shown that they have distinctly different duodenal microbial communities compared to healthy dogs. Current treatments for IBD include the administration of nonspecific anti-inflammatory drugs which may confer serious side effects and do not address the underlying basis for disease, namely, altered microbial composition. Use of probiotics (viable, non-pathogenic bacteria that exert health benefits beyond basic nutrition) offers an attractive, physiologic, and non-toxic alternative to shift the balance to protective species and treat IBD. The aim of the proposed study is to investigate the clinical, microbiologic, and anti-inflammatory effects of probiotic VSL#3 in the treatment of canine IBD. We hypothesize that VSL#3 used as an adjunct to standard therapy (i.e., elimination diet and prednisone) will induce a beneficial alteration of enteric bacteria leading to induction and maintenance of remission in dogs with IBD. A randomized, controlled clinical trial of 8 weeks duration is presently being performed to assess the efficacy of standard therapy + probiotic versus standard therapy alone.
Our data to date suggests that dogs treated with VSL#3 do respond favorably to the probiotic as evidenced by a reduction in their clinical disease severity. What remains unclear (ie, the clinicians are blinded as to whether a dog receives VSL#3 or placebo) is whether remission occurs more quickly in VSL#3-treated dogs vs placebo-medicated dogs. There is a need for additional data to be generated to provide proof of efficacy in probiotic therapy before these agents can be applied to widespread clinical use. These studies will also provide highly relevant insight into the anti-inflammatory effects of probiotics for treatment of human and canine IBD. We have produced 2 research abstracts to date with data from these studies.
We are actively seeking additional dogs for inclusion into the trial at this time, and would particularly welcome purebred dogs at increased risk for IBD including GSD, boxer, and French Bull dog breeds.
Grant Abstract: 1609 MY2 Summary
01759: Disrupting the Differentiation of Cancer Stem Cells to Prevent the Spread of Hemangiosarcoma
Grant Title: 01759: Disrupting the Differentiation of Cancer Stem Cells to Prevent the Spread of Hemangiosarcoma
Principal Investigator: Dr. Jaime F Modiano, VMD PhD
Research Institution: University of Minnesota
Start - End Dates: 1/1/2013 - 12/31/2015
Original Project Description:
Hemangiosarcoma is a rapidly fatal disease. The lifetime risk is alarmingly high for some breeds like Golden Retrievers (~20% will die of this disease) and Portuguese Water Dogs (~15% will die of this disease). Furthermore, the risk of hemangiosarcoma is not limited to a single breed. In fact so many dogs are at risk to develop hemangiosarcoma that 40 Breed Clubs designated it as a research priority for 2012. Despite considerable efforts to find effective treatments, the outcome for dogs with hemangiosarcoma has changed very little over the past 30 years. We believe this is because our understanding of this disease is still rudimentary, but that is changing. Recent evidence suggests hemangiosarcoma conforms to the "cancer stem cell" model, where a defined subset of cells is responsible for initiating and maintaining the tumor. These cells are resistant to conventional therapies and they also are very adaptable, being able to survive in a variety of niches. In the case of hemangiosarcoma, the cancer stem cells also retain or acquire the potential to differentiate along several different lineages. For this project, we will use this property against the tumor by modulating factors that support the self-renewal of the stem cell compartment and by inducing their terminal differentiation along alternate pathways that have reduced malignant potential. We propose that disrupting the interactions between hemangiosarcoma cancer stem cells and their microenvironment will enhance the sensitivity of these cells to conventional and targeted therapies and improve the outcomes of dogs with this disease.
Grant Objectives:
For this project, we will examine the potential to use the multipotency of hemangiosarcoma cells to our advantage by forcing them to differentiate into lineages with reduced malignant potential.
Report to Grant Sponsor from Investigator:
We continue on track to achieve the milestones laid out for this project. Our data continue to clarify the interactions stemming from inflammation in the tumor environment with regard to cell autonomous growth and survival, reinforcement of traits associated with metastasis, and interactions with other constituents of the microenvironment.
Grant Abstract: 1759 MY2 Summary
01787: Clinical Advancement of a Cancer Vaccine in Dogs
Grant Title: 01787: Clinical advancement of RNA-transfected CD40-B cell vaccine technology for cancer therapy
Principal Investigator: Dr. Nicola J Mason, BVetMed, PhD
Research Institution: University of Pennsylvania
Start - End Dates: 1/1/2013 - 12/31/2014
Original Project Description:
Canine lymphoma is the most common blood-based cancer in dogs with an estimated annual incidence of 30/100,000. Chemotherapy induces remission in 75-85% of patients; however, the majority of patients relapse with drug-resistant lymphoma within 8-10 months of diagnosis and most dogs die of their disease shortly thereafter. Cell-based vaccine strategies that stimulate anti-tumor immunity have shown promise in the treatment of many different cancer types including non-Hodgkin's lymphoma (NHL) in humans. In a previous study Dr. Mason developed a cell-based vaccine to induce anti-tumor immunity in dogs with NHL. Initial studies were hopeful as this early vaccine significantly prolonged second remission duration and overall survival, but ultimately the vaccine did not prevent relapse. These early findings suggest that while the lymphoma vaccine stimulated antitumor immunity it will require immunological boosting to achieve prolonged cancer-free survival. In the current study, Dr. Mason will optimize her cell-based vaccine approach to induce functional, long lasting tumor-specific immune responses that will prevent relapse and prolong survival in dogs with NHL.
Grant Objectives:
The goal is to optimize our vaccine/protocol to stimulate more effective anti-tumor immunity that will prevent relapse and prolong overall survival in dogs with NHL.
Report to Grant Sponsor from Investigator:
The goal of this proposal is to build on our previous work developing a cell-based vaccine that aims to stimulate potent tumor-specific immune responses that will kill lymphoma cancer cells. Our previous work has shown that white blood cells known as B cells found in the peripheral blood can be activated and grown outside of the body using special "feeder cells" that express an important molecule known as CD40L. The stimulated B cells (known as CD40-B cells) can be loaded with genetic material (RNA) that has been extracted from the patient's tumor. When re-injected back into the patient, the CD40-B cells are able to present the tumor material to the body's immune system and stimulate an anti-tumor immune response. We have shown in a phase I clinical trial that this approach has produced promising results with respect to prolonging overall survival in dogs with lymphoma. We have now improved the generation of this vaccine by generating canine specific feeder cells that are moderately more efficient at inducing canine B cells to grow from PBMCs. We have started to evaluate ways to improve the immune stimulatory function of these vaccine cells but have not yet identified an immune stimulant that can significantly augment the CD40-B cell capacity to stimulate canine T cells in vitro beyond that already achieved. Our studies in this area continue.
Our current methods of generating the CD40-B vaccine from lymphoma patients are laborintensive and require specialized laboratory equipment that is not available in most facilities. Therefore, we have made second-generation feeder cells that stably express the canine form of CD40L (we previously used the human CD40L molecule in our feeder cells) and have evaluated a non cell-based technique for canine B cell culture. We found that our second generation canine CD40L expressing feeder cells work well in our culture system and are much simpler to maintain in the laboratory than the previously used transfected cells expressing human CD40L. We also performed several experiments to evaluate whether these second-generation feeder cells can be irradiated, frozen and then thawed prior to their effective use in B cell generation. This would enable these cells to be distributed to other centers that do not have ready access to an irradiator and enable those centers to generate CD40-B cell vaccines on site. We have found that canine B cell expansion using thawed, previously irradiated KTcCD40L feeder cells is possible however it is sub-optimal when compared to freshly irradiated feeder cells. Unfortunately, we found that a soluble form of CD40L was not effective at activating and expanding canine B cells from peripheral blood lymphocytes in culture. Therefore, we will continue to generate CD40-B cell vaccines using freshly irradiated feeder cells.
We have also tested the hypothesis that the ability to stimulate anti-tumor immunity can be improved through the addition of a potent immune adjuvant (CpG DNA) to our CD40-B cell cultures. Our preliminary results indicate that while the addition of CpG may induce a mild increase in the number of CD40-B cells generated we have not identified any significant increase in expression of B cell surface molecules over and above a negative GpC control. These experiments are currently being repeated.
Regulatory approval for our second clinical trial using our improved CD40-B cell technology is being sought and we expect to begin recruitment for the second phase of this proposal in the near future.
Grant Abstract: 1787 EY1 Summary
01806: A Novel Virus-Based Anti-Tumor Treatment for Canine Osteosarcoma
Grant Title: 01806: A Novel Virus-Based Anti-Tumor Treatment for Canine Osteosarcoma
Principal Investigator: Dr. Bruce F Smith, VMD PhD
Research Institution: Auburn University
Start - End Dates: 3/1/2013 - 2/28/2015
Original Project Description:
Osteosarcoma is an aggressive canine bone cancer, accounting for around 6% of all canine cancers. Even with the standard-of-care therapy of amputation and chemotherapy, the prognosis is poor, with most dogs dying due to tumor spread (metastasis) within one year, and less than 20% surviving to 2 years following diagnosis. Therefore, improved strategies to treat metastatic disease are needed. In this respect, viruses can be engineered to multiply in, and kill, tumor cells and yet spare normal cells. We have developed a virus and have demonstrated that it can be both safely administered to patient dogs and have potential efficacy in treating osteosarcoma. While this virus was hypothesized to kill osteosarcoma cells through its replication, we have recently recognized the possibility that the virus stimulates an immune response to tumor, in addition to itself. In this study, we propose to examine the interaction of this virus with the immune system of dogs, including assessing any potential increase in immune response to the tumor. Patient dogs with a confirmed diagnosis of osteosarcoma will be treated with the virus following limb amputation, which will then be followed by 4-6 cycles of carboplatin chemotherapy. The dogs will be assessed for immune-responses to the virus and tumor, viral levels, and survival time.
Grant Objectives:
In this study, we propose to examine the interaction of this virus with the immune system of dogs, including assessing any potential increase in immune response to the tumor.
Report to Grant Sponsor from Investigator:
The clinical trial portion of this project has continued and 6 dogs have been enrolled (with an additional 2 pending). All 6 dogs have done well with the virus injection and have gone home as scheduled and no major side effects have been seen. All of the dogs have had their final blood draw (4 weeks post injection). One dog was euthanized shortly after its final blood draw due to orthopedic complications (cruciate tendon rupture) and a second was recently euthanized due to recurrent disease at 6 months post treatment at the site of limb sparing surgery. It is too soon to know how the remaining dogs have responded to the treatment, as we still need to run the appropriate assays and to monitor their progress. We have successfully harvested and grown osteosarcoma cells from every dog and we have validated most of the assays to be performed on the samples from these dogs. Experimental sample have begun to be assessed for virus quantitation (blood, urine, feces) and immunologic assays have been started for several parameters.
Grant Abstract: 1806 EY1 Summary
01771: Defining the Unique Genetic Markers in Dogs That Define Immune Function, Disease Resistance and Tissue Transplantation
Grant Title: 01771: Defining the Unique Genetic Markers in Dogs That Define Immune Function, Disease Resistance and Tissue Transplantation
Principal Investigator: Dr. Aravind Ramakrishnan, MD
Research Institution: Fred Hutchinson Cancer Research Center
Start - End Dates: 1/1/2013 - 12/31/2014
Original Project Description:
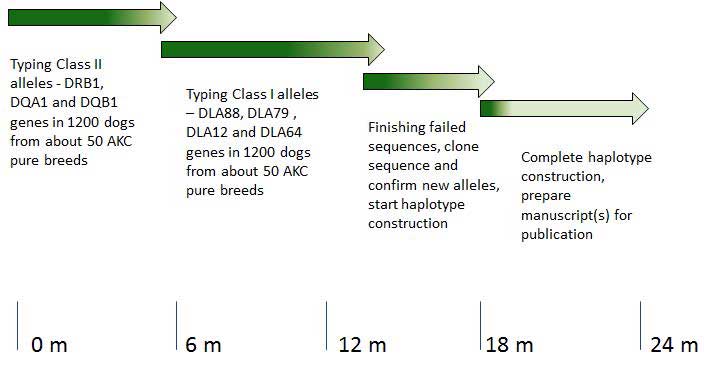
The Major Histocompatibility Complex (MHC) is a region of the genome that contains genes that code for a group of cell surface proteins known as Dog leukocyte antigens (DLA). DLA play important roles in the immune system including the recognition of self as well as recognition of foreign pathogens such as bacteria and viruses. The MHC genes are highly polymorphic and each gene has many different forms or alleles. Matching of MHC alleles between the donor and recipient is important for the success of stem cell and tissue transplants. Specific assortments of MHC alleles or haplotypes have been associated with an increased risk for the development of diabetes and auto immune diseases in humans. Knowledge of these associations has been valuable in understanding disease mechanisms. Recently we have developed improved methods for identifying the different forms of the DLA genes, in a large number of dogs of diverse breeds. In this application, we propose to characterize haplotypes, in over 1200 dogs from at least 50 breeds using a high throughput sequencing strategy. The distribution and frequency of different forms of each of these genes and their specific clustering among different breeds will greatly enhance our knowledge of the genetic diversity among breeds. The methodology and data gained from this study will enhance the power of association studies between MHC types and canine diseases. Such a database will also enable tissue transplantation from unrelated but matched donors as a treatment for advanced malignancies and other diseases, among dogs of most breeds.
Grant Objectives:
The goal of the project is to construct haplotypes of DLA alleles from about 1200 dogs of about 50 AKC pure breeds. The methodology and data gained from this study will identify the level of diversity of DLA alleles in different breeds and enhance the power of association studies between DLA haplotypes and canine diseases. Such a database will also enable tissue transplantation from unrelated but matched donors as a treatment for advanced malignancies and other diseases, among dogs of most breeds.
Report to Grant Sponsor from Investigator:
The Major Histocompatibility Complex (MHC) is a region of the genome in dogs that contains genes that encode a group of cell surface proteins known as Dog leukocyte antigens (DLA). DLA play important roles in the immune system including the recognition of self as well as recognition of foreign pathogens such as bacteria and viruses. The MHC genes are highly polymorphic i.e., each gene has many different forms or alleles. Matching of these MHC alleles between the donor and recipient is important for the success of treatments such as stem cell and organ transplants. Specific assortments of MHC alleles, comparable to 7 beads of different colors on a string, constitute a haplotype. Recently we have developed improved methods for identifying variants of the DLA genes, in a large number of dogs of diverse breeds. In this project, we proposed to characterize haplotypes, in over 1200 dogs from 50 pure AKC breeds using DNA sequencing.
The goal of the project is to construct haplotypes of DLA alleles from about 1200 dogs of about 50 AKC pure breeds. This can be divided into 3 major tasks.
- Typing the 4 polymorphic class I genes
- Typing the 3 polymorphic class II genes
- Cloning and/or confirming new and ambiguous alleles for all classI and II genes.
- Analyzing and deriving haplotype compositions
Aim 1 is about 90 % complete and Aim 2 is also about 90 % complete. Work on Aim 3 is in progress. Work on Aim 4 will commence shortly. Allele identities have been established for all the three class II genes, viz., DRB-1, DQA-1 and DQB-1 in over 900 dogs. Many new alleles have been found for all three genes. Allele identities have been established for two of the 4 class I genes, viz., DLA88, and DLA79 in over 900 dogs. Many new alleles have been found for both of these genes. Typing DLA64 and DLA12 genes is nearing completion. Haplotyping analysis will commence shortly.
Specific haplotypes have been associated with an increased risk for the development of diabetes and auto immune diseases in humans. Knowledge of these associations has been valuable in understanding disease mechanisms. The distribution and frequency of different forms of each of these genes and their groupings among different breeds will greatly enhance our knowledge of the genetic diversity among breeds. The methodology and data gained from this study will enhance the power of association studies between MHC types and canine diseases. For example, in collaboration with Dr. Leigh Anne Clark’s group at the Clemson University in South Carolina we have identified an association of a specific DLA class I allele with pancreatic acinar atrophy in the German Shepherd Dog. Such a database will also immediately enable tissue transplantation from unrelated but matched donors as a treatment for advanced malignancies and other diseases, among the dogs of most breeds.
We have achieved about 90 percent of the stated goals for this time, though we have changed our strategy. Work is being done in batch mode for logistics and cost efficiencies. Work is progressing very well and we do not anticipate any issues that will affect the successful completion of this project in time.
Grant Abstract: 1771 MY2 Summary
Development of Anti-IgE Peptide for Treatment of Canine Allergy
Grant Title: 01415: Development of Anti-IgE Peptide for Treatment of Canine Allergy
Principal Investigator: Dr. Bruce Hammerberg, DVM PhD
Research Institution: North Carolina State University
Start - End Dates: 1/1/2011 - 12/31/2012
Original Project Description:
Treatment of chronic allergic diseases in dogs, often seen as recurring dermatitis, frequently results in less than optimal outcomes. When the disease can be linked to exposure to specific allergens, such house dust mites, desensitization injections can be effective in some individuals when carried out over an extended time; however, most cases are not resolved by desensitization and require a combination of allergen avoidance and anti-inflammatory drugs. The prolonged use of these drugs, such as corticosteroids, can result in severe side effects. These same challenges exist for human allergy suffers, but recently there has been a major breakthrough in the development of a new, safe and effective therapy using a monoclonal antibody that specifically binds and neutralizes human IgE that is responsible for activating inflammation-producing cells. This new product is called Xolair® and it has been used safely by millions of allergy patients for more than 5 years. Our laboratory has developed a monoclonal antibody that specifically binds canine IgE in the same manner as the monoclonal antibody used to develop Xolair®. There are two obstacles remaining in providing the canine equivalent to Xolair® for treatment of allergies in dogs and they are the Objectives of this proposal: 1. Modifying the monoclonal antibody to reduce the dog's natural response to clear this protein; and, 2. Developing cost effective production of the modified antibody. Our Approach is to: 1. Generate a single chain recombinant peptide from the IgE-binding region of our canine IgE-specific monoclonal antibody that is small in size and of limited antigenic potential; and 2. Develop a transgenic plant (eg. tobacco) containing the gene for this recombinant peptide using well established techniques that will allow production of the therapeutic peptide in kilogram quantities. The expected outcome will be to provide a new, safe and highly effective treatment option for canine allergic diseases that is affordable to use for maintenance therapy.
Grant Objectives:
Objective 1: To create a recombinant, nonanaphylactic, single-chain antibody fragment (scFv) with high affinity for canine IgE from the variable region gene sequences of mAb 5.91 clones.
Objective 2: To generate a plant-derived recombinant, nonanaphylactic, single-chain antibody fragment with high affinity for IgE that can be scaled up for production at kilogram amounts.
Report to Grant Sponsor from Investigator:
1. To create a recombinant, non-anaphylactic, single-chain antibody fragment (scFv) with high affinity for canine IgE from the variable region gene sequences of mAb 5.91.
The sequence for the light chain variable region of mAb 5.91 was completed in April, 2011. The sequence for the heavy chain variable region was completed in October, 2011. Linkage of the two sequences and expression of a recombinant scFv of mAb 5.91 with confirmation of high affinity binding to canine IgE was completed in November, 2011.
A Fab fragment was produced from the whole molecule mAb 5.91 and used in flow cytometry assays as a model for the recombinant scFv version of the antibody by May, 2011. Whole blood from allergic dogs was processed and assayed. Results showed that the whole mAb 5.91 molecule reduced the amount of binding of canine IgE to the monocyte cell population from 15% to 7.7%. Moreover, the intact mAb 5.91 was able to bind the free IgE to prevent it from binding cell surface receptors. However, whole molecule mAb 5.91 complexed with canine IgE bound to 13.7% of the lymphocyte cell population possibly reacting with IgG Fc receptors. The Fab fragment of mAb 5.91, pre-incubated with canine IgE, reduced the binding of canine IgE to the monocyte cell population from 15% to 5.6%. This demonstrated that the Fab fragment of mAb 5.91 was even more effective in reducing the binding of IgE to the monocyte cell population than the intact mAb 5.91. There was no evidence of Fab fragment complexed with canine IgE binding to lymphocytes as previously seen with intact mAb 5.91.
These preliminary results indicate that the recombinant scFv form of the mAb 5.91 will be more effective at blocking IgE binding to cell surface receptors as well as decreasing the potential of cross reactivity of the lymphocyte cell population with the IgG Fc receptors than the original mAb 5.91.
2. To generate a plant-derived recombinant, nonanaphylactic, scFv with high affinity for IgE that can be scaled up for production at kilogram amounts. To be completed in the second year. Gene constructs of the newly made 5.91-scFv were designed to target the chloroplast and ER regions of the tobacco leaf cells. Both gene constructs were inserted into a PVX pGR106 amplicon vector and amplified in E.coli. The purified 5.91scFv-pGR106 constructs are being used to transform Agrobacterium tumefaciens strain GV3101. However, problems have been encountered during transformation attempts of Agrobacterium tumefaciens with the purified 5.91scFv-pGR106 constructs. A second round of transformation is being performed at this time.
TEV-B is a transgenic tobacco plant that expresses a mutated P1/HC-Pro suppressor of Post Transcriptional Gene Silencing. It has been shown that this line of tobacco plants produces higher protein yields than wild type varieties of tobacco including Nicotiana benthamiana. TEVB seeds were planted on May 23rd and TEV-B plants should be ready for infection in July. TEV-B plants were Agroinfected with Agrobacterium tumefaciens GV3101 containing the gene 5.91scFv::chloro gene construct. Total protein was extracted from 2kg of transgenic leaf tissue. Crude extract was clarified from photosynthetic proteins and polyphenols that may interact with downstream applications.
Binding activity of the 5.91scFv in the extract was confirmed on ELISA and was later compared to the activity of 5.91Fab and intact mAb 5.91 in whole blood and canine mast cells flow cytometry assays.
Grant Progress Report: 1415 EY2 FINAL Summary
Identification and Characterization of a Canine Derived Single Chain Antibody that Binds and Neutralizes Canine VEGF
Grant Title: 01484: Identification and Characterization of a Canine Derived Single Chain Antibody that Binds and Neutralizes Canine VEGF
Principal Investigator: Dr. Nicola J Mason, BVetMed, PhD
Research Institution: University of Pennsylvania
Start - End Dates: 1/1/2011 - 6/30/2012
Original Project Description:
Canine hemangiosarcoma (HSA) is a common and highly aggressive tumor of blood vessels that is oftentimes fatal. At diagnosis most dogs have evidence of metastatic disease and despite chemotherapy, survival times rarely exceed 6 months. Novel approaches to the treatment of this disease are needed. The use of targeting antibodies against vascular endothelial growth factor (VEGF), a protein that promotes tumor growth and spread, plus chemotherapy has prolonged disease free survival in several different tumor types in man. It is the aim of this proposal to isolate a canine derived antibody fragment that can specifically bind to and neutralize the tumor promoting effects of canine VEGF. It is hypothesized that a VEGF specific antibody fragment that is canine in origin may be used in the veterinary clinics to retard or prevent the development of metastases in dogs with HSA. We have generated diverse libraries of canine antibody fragments that we will screen against canine VEGF to select fragments that specifically recognize canine VEGF. We will isolate VEGF specific antibody fragments using previously described techniques that are routinely performed in our laboratory and test their ability to inhibit the tumor promoting effects of VEGF in vitro. This work will build on our previous studies supported by the CHF that describe the technology to generate canine antibody fragment libraries. This canine-derived, tumor-specific targeting approach is the first of its kind in the veterinary field and if successful this agent may also be used to treat many other tumor types in the dog.
Grant Objectives:
Objective 1: To isolate canine derived scFvs that bind specifically to canine VEGF
Objective 2: To isolate VEGF-specific scFvs that inhibit VEGF bioactivity.
Report to Grant Sponsor from Investigator:
In this proposal we set out to utilize our canine-derived scFv phage display platform technology to isolate canine antibody fragments that bind and neutralize Vascular Endothelial Growth Factor (VEGF). VEGF is a protein growth factor that promotes the growth and survival of new blood vessels. Tumors require the growth of new blood vessels to supply them with oxygen and nutrients that allow the tumor to grow. VEGF is a major contributor to this angiogenesis (growth of new blood vessels).
Bevacizumab (Avastin) is a humanized antibody that recognizes and neutralizes human VEGF. It is currently approved for the treatment of a number of different cancers including metastatic colon cancer, and non-small cell lung cancer. FDA approval and on-going clinical trials attest to the potential of VEGF inhibition as a treatment for many different cancer types in humans, however, the therapeutic effects of VEGF neutralization on cancer growth and metastasis have not been evaluated in the dog. Expression of VEGF has been reported in a wide range of different tumor types in the dog including hemangiosarcoma, malignant melanoma, soft tissue sarcomas, mast cell tumors, nasal carcinomas, intracranial neoplasias, and simple mammary gland adenocarcinomas and inflammatory mammary carcinoma. Serum levels of VEGF are increased in dogs with osteosarcoma, malignant melanoma and HSA and in dogs with osteosarcoma and malignant melanoma, serum levels correlate with disease free interval and survival times respectively. Together these reports suggest that VEGF plays an important role in the progression of these tumor types in the dog and as such represents a potential therapeutic target. However, to date there are no canine-derived antibodies that bind and neutralize the angiogenic activity of canine VEGF.
During this project, we identified 3 canine-derived scFv or antibody fragments that bind to canine VEGF. Furthermore results from cell-based assays in vitro suggest that these antibody fragments might inhibit the growth stimulating effects of VEGF on healthy endothelial cells and on a canine hemangiosarcoma cell line in vitro. These results suggest that not only do these antibody fragments bind VEGF but they also might inhibit its biological activity, which would be essential for any therapeutic effect. We have since developed several sophisticated techniques including surface plasmon resonance (SPR) to more closely interrogate the ability of our isolated antibody fragments to neutralize the activity of VEGF (i.e. inhibit the ability of VEGF to interact with a key VEGF receptor). Disappointingly, the results of these experiments have shown that the most abundant antibody fragment that we isolated from our library does not appear to affect the interaction of VEGF with its receptor suggesting that this clone would not inhibit the biological effects of VEGF on tumor blood vessels. However, we are now testing the remaining 2 isolated scFv using these advanced techniques to determine whether they can neutralize VEGF.
In summary, this work has 1) demonstrated that canine derived antibody fragments that bind to VEGF can be isolated from libraries using simple panning techniques; and 2) optimized sophisticated techniques (surface plasmon resonance) that we can now use to determine whether antibody fragments interact with molecules of interest and can inhibit particular molecular interactions in vitro prior to their translation into patients with diseases such as hemangiosarcoma. We will continue to utilize these techniques to evaluate the remaining 2 scFv. If we find that one or both of these scFv neutralize VEGF activity then we intend to identify commercial partners that will assist in generating canine monoclonal antibodies based on these scFv that could then enter clinical trials in patients with hemangiosarcoma or other tumor types that are associated with excess production of VEGF.
Grant Progress Report: 1484 MY2 FINAL Summary